 | |
| Clinical data | |
|---|---|
| Other names | AZD0914; ETX0914 |
| Pregnancy category |
|
| Routes of administration | Oral |
| Drug class | Antibiotic |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 97.8% |
| Metabolism | Hepatic |
| Onset of action |
|
| Elimination half-life | 5.3–6.3 h |
| Excretion | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
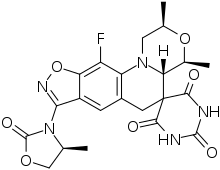
| Formula | C22H22FN5O7 |
| Molar mass | 487.444 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Zoliflodacin (development codes AZD0914 and ETX0914) is an experimental antibiotic that is being studied for the treatment of infection with Neisseria gonorrhoeae (gonorrhea).[1] It has a novel mechanism of action which involves inhibition of bacterial type II topoisomerases.[2] Zoliflodacin is being developed by Innoviva Specialty Therapeutics, and the drug has demonstrated clinical efficacy equivalent to ceftriaxone in Phase III clinical trials.[3][4]
Susceptible bacteria
Zoliflodacin has shown in vitro activity[5] against the following species of bacteria:
Pharmacology
Mechanism of action
Zoliflodacin is primarily active against both Gram-positive, but has activity against fastidious Gram-negative bacteria. It functions by inhibiting DNA gyrase, an enzyme necessary to separate bacterial DNA, thereby inhibiting cell replication.
History
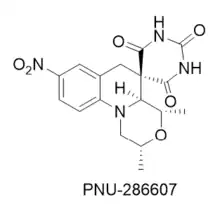
A high throughput screening campaign aimed at identifying compounds with whole cell antibacterial activity performed at Pharmacia & Upjohn identified compound PNU-286607, a progenitor of Zoliflodacin, as having the desired activity.[6] Subsequent biological profiling of PNU-286607 showed that the compound inhibited DNA synthesis in susceptible bacteria, and analysis of mutants resistant to the compound's activity indicated that these compounds acted on DNA gyrase at a site distinct from that of the fluoroquinolone antibiotics.
Subsequent research at AstraZeneca led to the discovery that the nitroaromatic in PNU-286607 could be replaced with a fused benzisoxazole ring,[7] which allowed for an exploration of different groups at the 3-position of the heterocycle. This work was continued at Entasis Pharmaceuticals where extensive optimization resulted in the discovery of ETX0914,[8] which was renamed Zolifodacin in the course of its clinical development.
References
- ↑ Taylor SN, Marrazzo J, Batteiger BE, Hook EW, Seña AC, Long J, et al. (November 2018). "Single-Dose Zoliflodacin (ETX0914) for Treatment of Urogenital Gonorrhea". The New England Journal of Medicine. 379 (19): 1835–1845. doi:10.1056/NEJMoa1706988. hdl:1805/19865. PMID 30403954.
- ↑ Basarab GS, Kern GH, McNulty J, Mueller JP, Lawrence K, Vishwanathan K, et al. (July 2015). "Responding to the challenge of untreatable gonorrhea: ETX0914, a first-in-class agent with a distinct mechanism-of-action against bacterial Type II topoisomerases". Scientific Reports. 5: 11827. Bibcode:2015NatSR...511827B. doi:10.1038/srep11827. PMC 4501059. PMID 26168713.
- ↑ "GARDP and Innoviva Specialty Therapeutics Announce Completion of Patient Recruitment for Registrational Phase 3 Gonorrhea Treatment Trial". Innoviva Specialty Therapeutics. 23 May 2023. Retrieved 6 November 2023.
- ↑ "Positive Results Announced in Largest Pivotal Phase 3 Trial of a First-In-Class Oral Antibiotic to Treat Uncomplicated Gonorrhea". Global Antibiotic Research & Development Partnership. 2023-11-01. Retrieved 2023-11-03.
- ↑ Basarab GS, Kern GH, McNulty J, Mueller JP, Lawrence K, Vishwanathan K, et al. (July 2015). "Responding to the challenge of untreatable gonorrhea: ETX0914, a first-in-class agent with a distinct mechanism-of-action against bacterial Type II topoisomerases". Scientific Reports. 5 (1): 11827. Bibcode:2015NatSR...511827B. doi:10.1038/srep11827. PMC 4501059. PMID 26168713.
- ↑ Miller AA, Bundy GL, Mott JE, Skepner JE, Boyle TP, Harris DW, et al. (August 2008). "Discovery and characterization of QPT-1, the progenitor of a new class of bacterial topoisomerase inhibitors". Antimicrobial Agents and Chemotherapy. 52 (8): 2806–2812. doi:10.1128/AAC.00247-08. PMC 2493097. PMID 18519725.
- ↑ Basarab GS, Brassil P, Doig P, Galullo V, Haimes HB, Kern G, et al. (November 2014). "Novel DNA gyrase inhibiting spiropyrimidinetriones with a benzisoxazole scaffold: SAR and in vivo characterization". Journal of Medicinal Chemistry. 57 (21): 9078–9095. doi:10.1021/jm501174m. PMID 25286019.
- ↑ Basarab GS, Kern GH, McNulty J, Mueller JP, Lawrence K, Vishwanathan K, et al. (July 2015). "Responding to the challenge of untreatable gonorrhea: ETX0914, a first-in-class agent with a distinct mechanism-of-action against bacterial Type II topoisomerases". Scientific Reports. 5 (1): 11827. Bibcode:2015NatSR...511827B. doi:10.1038/srep11827. PMC 4501059. PMID 26168713.