 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
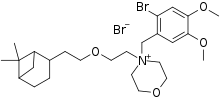
| Formula | C26H41Br2NO4 |
| Molar mass | 591.425 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Pinaverium bromide (INN) is a medication used for functional gastrointestinal disorders. It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping to restore the normal contraction process of the bowel. It is most effective when taken for a full course of treatment and is not designed for immediate symptom relief or sporadic, intermittent use.
Pinaverium bromide was first registered in 1975 by Solvay Pharmaceuticals (now a division of Abbott Laboratories), and marketed globally using the brand names Dicetel and Eldicet. Generic pinaverium is available in South Korea under a trade name of Disten[1] and in Argentina as Nulite.[2]
Indications
It is indicated for the treatment and relief of symptoms associated with irritable bowel syndrome (IBS) including abdominal pain, bowel disturbances and intestinal discomfort; and treatment of symptoms related to functional disorders of biliary tract.[3]
References
- ↑ KMLE 약품/의약품 정보: 디스텐정 (Disten Tab.) (in Korean)
- ↑ "NULITE | Laboratorio Dominguez".
- ↑ Abbott Laboratories (Feb 2012). Dicetel Product Insert/Information Malaysia.