 | |
| Clinical data | |
|---|---|
| Trade names | Qbrexza, Rapifort |
| Other names | Glycopyrronium tosilate hydrate (JAN JP) |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | Topical |
| Drug class | Muscarinic antagonist |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| 3D model (JSmol) | |
| |
| |
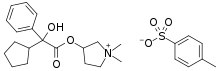
Glycopyrronium tosylate, sold under the brand name Qbrexza among others, is a medication used for the treatment of primary axillary hyperhidrosis.[1][2][3]
It was approved for medical use in the United States in June 2018,[4] and in Japan in January 2022.[5]
References
- 1 2 "Qbrexza- glycopyrronium cloth". DailyMed. 17 January 2022. Retrieved 2 November 2022.
- ↑ Nwannunu CE, Limmer AL, Coleman K, Shah R, Patel RR, Mui UN, Tyring SK (March 2019). "Glycopyrronium Tosylate (Qbrexza) for Hyperhidrosis". Skin Therapy Letter. 24 (2): 1–3. PMID 30970203.
- ↑ Lamb YN (November 2019). "Topical Glycopyrronium Tosylate in Primary Axillary Hyperhidrosis: A Profile of Its Use". Clinical Drug Investigation. 39 (11): 1141–1147. doi:10.1007/s40261-019-00853-x. PMC 6877702. PMID 31571127.
- ↑ "Drug Approval Package: Qbrexza (glycopyrronium)". U.S. Food and Drug Administration (FDA). 20 November 2018. Retrieved 1 November 2022.
- ↑ "Maruho Launches Primary Axillary Hyperhidrosis Treatment". Maruho. 23 May 2022. Retrieved 2 November 2022.
External links
- "Glycopyrronium tosylate". Drug Information Portal. U.S. National Library of Medicine.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.