 | |
| Names | |
|---|---|
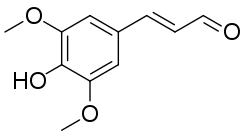
| Systematic IUPAC name
(E)-3-(4-Hydroxy-3,5-dimethoxyphenyl)prop-2-enal[1] | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| 2215799 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.156.065 |
| EC Number |
|
| KEGG | |
| MeSH | Sinapaldehyde |
PubChem CID |
|
| |
| |
| Properties | |
| C11H12O4 | |
| Molar mass | 208.213 g·mol−1 |
| Melting point | 104 to 106 °C (219 to 223 °F; 377 to 379 K) |
| log P | 1.686 |
| Acidity (pKa) | 9.667 |
| Basicity (pKb) | 4.330 |
| Hazards | |
| GHS labelling:[2] | |
 | |
| Warning | |
| H315, H319, H335 | |
| Related compounds | |
Related alkenals |
Cinnamaldehyde |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Sinapaldehyde is an organic compound with the formula HO(CH3O)2C6H2CH=CHCHO. It is a derivative of cinnamaldehyde, featuring one hydroxy group and two methoxy groups as substituents. It is an intermediate in the formation of sinapyl alcohol, a lignol that is a major precursor to lignin.[3][4]
Biosynthetic role
In sweetgum (Liquidambar styraciflua), sinapaldehyde arises in two steps from coniferyl aldehyde beginning with hydroxylation mediated by coniferyl aldehyde 5-hydroxylase. The diphenol is then methylated at the 5-OH by the action of caffeate O-methyltransferase.[5]
Sinapaldehyde is reduced to the alcohol by the action of dehydrogenase enzymes.[4] In Arabidopsis thaliana, the enzyme dihydroflavonol 4-reductase uses NADP+ to reduce sinapaldehyde to sinapyl alcohol.[6]
It is found in Senra incana (Hibisceae). It is a low molecular weight phenol that is susceptible to extraction from cork stoppers into wine.[7]
See also
References
- ↑ "AC1L3OEQ - Compound Summary". The PubChem Project. USA: National Center for Biotechnology Information.
- ↑ "C&L Inventory". www.echa.europa.eu.
- ↑ Wout Boerjan, John Ralph, Marie Baucher "Lignin Biosynthesis" Annu. Rev. Plant Biol. 2003, vol. 54, pp. 519–46. doi:10.1146/annurev.arplant.54.031902.134938
- 1 2 Li, Laigeng; Cheng, Xiao Fei; Leshkevich, Jacqueline; Umezawa, Toshiaki; Harding, Scott A.; Chiang, Vincent L. (2001). "The Last Step of Syringyl Monolignol Biosynthesis in Angiosperms is Regulated by a Novel Gene Encoding Sinapyl Alcohol Dehydrogenase". The Plant Cell. 13 (7): 1567–1586. doi:10.1105/tpc.010111. PMC 139549. PMID 11449052.
- ↑ Osakabe, Keishi; Tsao, Cheng Chung; Li, Laigeng; Popko, Jacqueline L.; Umezawa, Toshiaki; Carraway, Daniel T.; Smeltzer, Richard H.; Joshi, Chandrashekhar P.; Chiang, Vincent L. (1999). "Coniferyl aldehyde 5-hydroxylation and methylation direct syringyl lignin biosynthesis in angiosperms". Proceedings of the National Academy of Sciences. 96 (16): 8955–8960. Bibcode:1999PNAS...96.8955O. doi:10.1073/pnas.96.16.8955. PMC 17714. PMID 10430877.
- ↑ Dihydroflavonol 4-reductase on arabidopsisreactome.org
- ↑ Polyphenolic Composition of Quercus suber Cork from Different Spanish Provenances. Elvira Conde, Estrella Cadahía, María Concepción García-Vallejo and Brígida Fernández de Simón, J. Agric. Food Chem., 1998, volume 46, pp 3166–3171 doi:10.1021/jf970863k