| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2,6-Dichlorophenol | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.602 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2020 2021 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H4Cl2O | |||
| Molar mass | 163.00 g·mol−1 | ||
| Appearance | white solid | ||
| Density | 1.653 g/cm3 at 20 °C[2] | ||
| Melting point | 66.6 °C (151.9 °F; 339.8 K)[2] | ||
| Boiling point | 226 °C (439 °F; 499 K)[2] | ||
| Hazards | |||
| GHS labelling: | |||
   | |||
| Danger | |||
| H314, H315, H319, H411 | |||
| P260, P264, P273, P280, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P332+P313, P337+P313, P362, P363, P391, P405, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
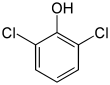
2,6-Dichlorophenol is a compound with formula C6H3Cl2OH. It is one of the six isomers of dichlorophenol. It is a colorless solid. Its pKa is 6.78, which is about 100x more acidic than 2-chlorophenol (8.52) and 1000x more acidic than phenol itself (9.95).[3]
Preparation
It can be produced in a multistep process from phenol, which is converted to its 4-sulfonic acid derivative. The resulting phenol sulfonic acid chlorinates at the positions flanking the phenol. Hydrolysis releases the sulfonic acid group.[4]
An alternative synthesis starts with the ethyl ester of 4-hydroxybenzoic acid, which chlorinates at the positions flanking the phenolic center. Ester hydrolysis followed by decarboxylation affords 2,6-dichlorophenol.[5]
References
- ↑ 2,6-Dichlorophenol at Sigma-Aldrich
- 1 2 3 Haynes, p. 3.166
- ↑ François Muller; Liliane Caillard (2011). "Chlorophenols". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a07_001.pub2. ISBN 978-3527306732.
- ↑ Otto Lindner; Lars Rodefeld (2005). "Benzenesulfonic Acids and Their Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_507. ISBN 978-3527306732.
- ↑ D. S. Tarbell; J. W. Wilson; Paul E. Fanta (1949). "2,6-Dichlorophenol". Org. Synth. 29: 35. doi:10.15227/orgsyn.029.0035.
Cited sources
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.