 | |
| Names | |
|---|---|
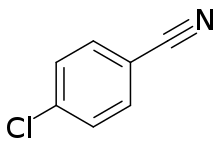
| IUPAC name
4-Chlorobenzonitrile | |
| Other names
p-Chlorobenzonitrile | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.009.788 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H4ClN | |
| Molar mass | 137.57 g·mol−1 |
| Appearance | white solid |
| Melting point | 97 °C (207 °F; 370 K) |
| Hazards | |
| GHS labelling:[1] | |
  | |
| Danger | |
| H302, H311, H319, H332, H412 | |
| P261, P264, P270, P271, P273, P280, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P361, P362, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
4-Chlorobenzonitrile is an organic compound with the formula ClC6H4CN. It is a white solid. The compound, one of three isomers of chlorobenzonitrile, is produced industrially by ammoxidation of 4-chlorotoluene. The compound is of commercial interest as a precursor to pigments.[2]
References
- ↑ "4-Chlorobenzonitrile". pubchem.ncbi.nlm.nih.gov.
- ↑ Pollak, Peter; Romeder, Gérard; Hagedorn, Ferdinand; Gelbke, Heinz-Peter (2000). "Nitriles". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_363. ISBN 978-3527306732.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.