 | |
| Names | |
|---|---|
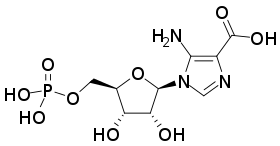
| IUPAC name
5-Amino-1-(5-O-phosphono-β-D-ribofuranosyl)-1H-imidazole-4-carboxylic acid | |
| Systematic IUPAC name
5-Amino-1-{(2R,3R,4S,5R)-3,4-dihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl}-1H-imidazole-4-carboxylic acid | |
| Other names
Carboxyaminoimidazole ribotide, Carboxyaminoimidazole ribonucleotide, CAIR, | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H14N3O9P | |
| Molar mass | 339.196 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
5′-Phosphoribosyl-4-carboxy-5-aminoimidazole (or CAIR) is an intermediate in the formation of purines.
It is formed by phosphoribosylaminoimidazole carboxylase.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.