| Identifiers | |
|---|---|
| |
3D model (JSmol) |
|
| 1869102 | |
| ChEBI |
|
| ChEMBL |
|
| ChemSpider | |
| EC Number |
|
| 185412 | |
| KEGG |
|
PubChem CID |
|
| UNII |
|
| |
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Anthrols (sometimes called anthranols) are the hydroxylated derivatives of anthracene. For the monohydroxo derivatives, three isomers are possible: 1-anthrol, 2-anthrol, and 9-anthrol. The latter exists as a minor tautomer of 9-anthrone.
| Name | CAS | Melting point | Structure | |
|---|---|---|---|---|
| 1-anthrol, 1-hydroxyanthracene | 610-50-4 | 150 °C | 302 °F | 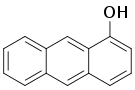 |
| 2-anthrol, 2-hydroxyanthracene | 613-14-9 | 166 °C | 331 °F | 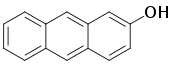 |
| 9-anthrol, 9-hydroxyanthracene[1] | 529-86-2 | 152 °C | 306 °F | 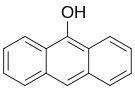 |
References
- ↑ Ośmiałowski, Borys; Raczyńska, Ewa D.; Krygowski, Tadeusz M. (2006). "Tautomeric Equilibria and Pi Electron Delocalization for Some Monohydroxyarenes Quantum Chemical Studies". The Journal of Organic Chemistry. 71 (10): 3727–3736. doi:10.1021/jo052615q. PMID 16674042.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.