 | |
| Names | |
|---|---|
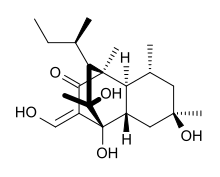
| IUPAC name
(3Z)-4,6,9-trihydroxy-3-(hydroxymethylidene)-1,6,8,9-tetramethyl-10-(1-methylpropyl)octahydro-1,4-ethanonaphthalen-2(1H)-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H34O5 | |
| Molar mass | 366.498 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
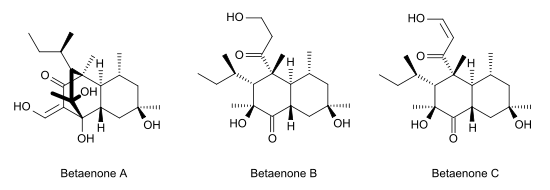
Betaenone A, like other betaenones (B and C), is a secondary metabolite isolated from the fungus Pleospora betae, a plant pathogen.[1] Of the seven phytotoxins isolated in fungal leaf spots from sugar beet (Beta vulgaris), it showed 73% growth inhibition.[2]
References
- ↑ Ichihara A.; Oikawa, Hideaki; Hayashi, Kazuko; Sakamura, Sadao; Furusaki, Akio; Matsumoto, Takeshi (1983). "Structures of Betaenones A and B, Novel Phytotoxins from Phoma betae Fr". J. Am. Chem. Soc. 105 (9): 2907–2908. doi:10.1021/ja00347a070.
- ↑ Haraguchi, T.; Oguro, Mieko; Nagano, Hiroshi; Ichihara, Akitami; Sakamura, Sadao (1983). "Specific inhibitors of eukaryotic DNA synthesis and DNA polymerase α, 3-deoxyaphidicolin and aphidicolin-17-monoacetate". Nucleic Acids Res. 11 (4): 1197–2000. doi:10.1093/nar/11.4.1197. PMC 325786. PMID 6402759.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.