 | |
| Names | |
|---|---|
| Preferred IUPAC name
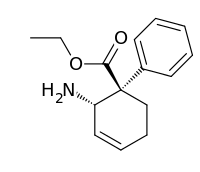
Ethyl (1R,2S)-2-amino[1,1′-bi(cyclohexane)]-1′,3,3′,5′-tetraene-1-carboxylate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H19NO2 | |
| Molar mass | 245.322 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Bisnortilidine is an opioid metabolite.[1] It is formed from tilidine by demethylation in the liver.
References
- ↑ Wustrow, I.; Riedel, K. D.; Mikus, G.; Weiss, J. (2012). "In vitro identification of the cytochrome P450 isozymes involved in the N-demethylation of the active opioid metabolite nortilidine to bisnortilidine". Naunyn-Schmiedeberg's Archives of Pharmacology. 385 (6): 633–9. doi:10.1007/s00210-012-0737-z. PMID 22349139. S2CID 2660215.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.