| Names | |
|---|---|
| IUPAC name
Copper(II) selenite | |
| Other names
Copper selenite dihydrate | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.030.465 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Appearance | Blue[1] |
| Solubility | Insoluble in water[1] Slightly soluble in benzene and acids[1] |
| Hazards | |
| GHS labelling: | |
 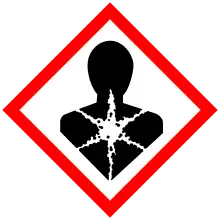  | |
| Danger | |
| H301, H331, H371, H410 | |
| P260, P261, P264, P270, P271, P273, P301+P310, P304+P340, P311, P314, P321, P330, P391, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Copper(II) selenite is an inorganic salt frequently found as its dihydrate, CuSeO3·2H2O, in the form of a blue powder.[2][3]
Preparation
Copper(II) selenite can be prepared from copper(II) acetate and selenous acid.[2][4]
Uses
Copper(II) selenite can be used a catalyst for Kjeldahl digestion.[2]
See also
References
- 1 2 3 "Copper(II) selenite". Sigma-Aldrich.
- 1 2 3 "Copper Selenite as a catalyst in the Kjeldahl nitrogen determination". Department of Chemistry, University of Wisconsin, Madison. November 1935.
- ↑ "Sigma Aldrich - Copper(II) selenite dihydrate".
- ↑ Hurd, Loren C.; Kemmerer, George I.; Meloche, V. W. (2002-05-01). "The Ammonates of Copper Selenite1". Journal of the American Chemical Society. 52 (10): 3881–3886. doi:10.1021/ja01373a018.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.