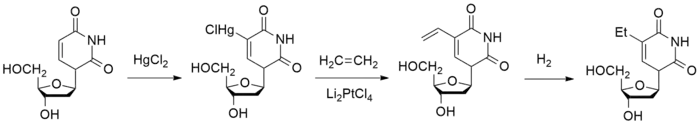 | |
| Clinical data | |
|---|---|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.035.645 |
| Chemical and physical data | |
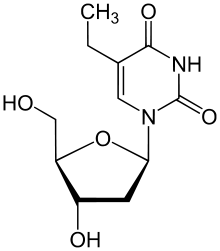
| Formula | C11H16N2O5 |
| Molar mass | 256.25514 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Edoxudine (or edoxudin) is an antiviral drug. It is an analog of thymidine, a nucleoside.
It has shown effectiveness against herpes simplex virus.[1]
Synthesis
Mercuration of the 2'-deoxyuridine 1 leads to the organometallic derivative 2; reaction of that with ethylene in the presence dilithiopalladium tetrachloride gives the alkylation product 3; this is reduced catalytically in situ. There is thus obtained the antiviral agent edoxudine 4.
References
- ↑ Hamuy R, Berman B (December 1998). "Topical antiviral agents for herpes simplex virus infections". Drugs of Today. 34 (12): 1013–1025. doi:10.1358/dot.1998.34.12.487486. PMID 14743269.
- ↑ US 3553192, Gauri KK, issued 1971, assigned to Robugen
- ↑ Bergstrom DE, Ruth JL (March 1976). "Letter: Synthesis of C-5 substituted pyrimidine nucleosides via organopalladium intermediates". Journal of the American Chemical Society. 98 (6): 1587–1589. doi:10.1021/ja00422a056. PMID 1249369.
- ↑ Bergstrom DE, Ogawa MK (1978). "C-5 substituted pyrimidine nucleosides. 2. Synthesis via olefin coupling to organopalladium intermediates derived from uridine and 2'-deoxyuridine". Journal of the American Chemical Society. 100 (26): 8106–8112. doi:10.1021/ja00494a014.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.