 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.153.466 |
| EC Number |
|
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C72H61P4Rh | |
| Molar mass | 1153.12 |
| Appearance | yellow solid |
| Density | 1.328 g/cm3 |
| Melting point | 162–163 °C (324–325 °F; 435–436 K) |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
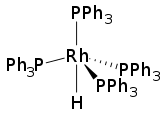
Hydridotetrakis(triphenylphosphine)rhodium(I) is the coordination complex with the formula HRh[P(C6H5)3]4. It consists of a Rh(I) center complexed to four triphenylphosphine (PPh3) ligands and one hydride. The molecule has idealized C3v symmetry.[1] The compound is a homogeneous catalyst for hydrogenation and related reactions.[2] It is a yellow solid that dissolves in aromatic solvents.
Preparation
In the presence of base, H2, and additional triphenylphosphine, Wilkinson's catalyst (chloridotris(triphenylphosphane)rhodium(I)) converts to HRh(PPh3)4:[3]
- RhCl(PPh3)3 + H2 + KOH + PPh3 → RhH(PPh3)4 + H2O + KCl
References
- ↑ Baker, R. W.; Pauling, Peter (1969). "The crystal and molecular structure of tetrakistriphenylphosphinerhodium(I) hydride". Journal of the Chemical Society D: Chemical Communications (24): 1495. doi:10.1039/c29690001495.
- ↑ Eduardo Peña-Cabrera "Hydridotetrakis(triphenylphosphine)rhodium" Encyclopedia of Reagents for Organic Synthesis, 2001, John Wiley & Sons. doi:10.1002/047084289X.rh030m
- ↑ Ahmad, N.; Levison, J. J.; Robinson, S. D.; Uttley, M. F. (1990). "Hydrido Phosphine Complexes of Rhodium(I)". Inorganic Syntheses. Vol. 28. pp. 81–83. doi:10.1002/9780470132593.ch19. ISBN 9780470132593.
{{cite book}}:|journal=ignored (help)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.