 | |
| Clinical data | |
|---|---|
| Other names | Lu-AE-58054 |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEMBL | |
| ECHA InfoCard | 100.245.270 |
| Chemical and physical data | |
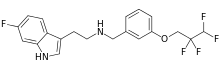
| Formula | C20H19F5N2O |
| Molar mass | 398.377 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Idalopirdine (INN) (code names Lu AE58054,) is a potent and selective 5-HT6 receptor antagonist under development by Lundbeck as an augmentation therapy for the treatment of cognitive deficits associated with Alzheimer's disease and schizophrenia.[1][2] As of October 2013 it is in phase III clinical trials.[2]
A phase III trial of two different daily doses of Lu AE58054 on top of 10 mg of donepezil for mild-to-moderate Alzheimer's failed to meet its primary endpoint with either dose.[3] Two further phase III trials failed too, the company confirmed in early 2017.[3]
See also
References
- ↑ "U.S. Development Programs". Lundbeck.
- 1 2 "Search of: Lu AE58054 - List Results". ClinicalTrials.gov. National Library of Medicine, U.S. Department of Health and Human Services.
- 1 2 Taylor NP (23 September 2016). "PhIII Alzheimer's flop takes chunk out of Lundbeck, hits Axovant with aftershocks".
External links
- Lundbeck expands its pipeline - initiating phase II clinical trials with a new compound for the treatment of schizophrenia Archived 2011-07-09 at the Wayback Machine
- Lundbeck initiates clinical phase II trials with Lu AE58054 as augmentation treatment in Alzheimer's disease Archived 2010-02-04 at the Wayback Machine
| AChE inhibitor medications | |
|---|---|
| Other medications | |
| Experimental BACE inhibitors | |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.