 | |
| Names | |
|---|---|
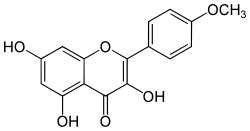
| IUPAC name
3,5,7-Trihydroxy-4′-methoxyflavone | |
| Systematic IUPAC name
3,5,7-Trihydroxy-2-(4-methoxyphenyl)-4H-1-benzopyran-4-one | |
| Other names
Kaempferid 4′-Methylkaempferol Kaempferol 4′-methyl ether 4′-O-Methylkaempferol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.036 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H12O6 | |
| Molar mass | 300.26 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Kaempferide is an O-methylated flavonol, a type of chemical compound. It can be found in Kaempferia galanga (aromatic ginger). It has been noted to inhibit pancreatic cancer growth by blockading an EGFR-related pathway.[1]
Metabolism
The enzyme kaempferol 4'-O-methyltransferase uses S-adenosyl-L-methionine and kaempferol to produce S-adenosyl-L-homocysteine and kaempferide.
Glycosides
Icariin is the tert-amyl alcohol derivative of kaempferide 3,7-O-diglycoside.
References
- ↑ Lee, Jungwhoi; Kim, Jae Hoon (2016). "Kaempferol Inhibits Pancreatic Cancer Cell Growth and Migration through the Blockade of EGFR-Related Pathway In Vitro". PLOS ONE. 11 (5): e0155264. Bibcode:2016PLoSO..1155264L. doi:10.1371/journal.pone.0155264. PMC 4866780. PMID 27175782.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.