 | |
| Clinical data | |
|---|---|
| Trade names | Lacipil, Motens |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~10% |
| Protein binding | >95% |
| Metabolism | Hepatic |
| Onset of action | 30–50 min |
| Elimination half-life | 13–19 hours |
| Excretion | Feces (~70%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.166.373 |
| Chemical and physical data | |
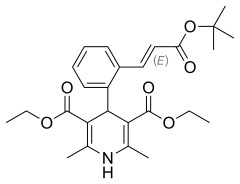
| Formula | C26H33NO6 |
| Molar mass | 455.551 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Lacidipine (tradenames Lacipil or Motens) is a calcium channel blocker. It is available as tablets containing 2 or 4 mg.
It was patented in 1984 and approved for medical use in 1991.[1]
References
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 466. ISBN 9783527607495.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.