 | |
 | |
| Names | |
|---|---|
| IUPAC name
Lyxose | |
| Systematic IUPAC name
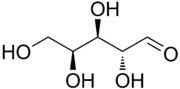
(2R,3R,4S)-2,3,4,5-Tetrahydroxypentanal | |
| Other names
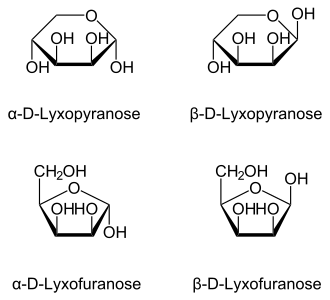
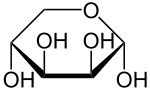
L-Lyxose Lyxopyranose | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C5H10O5 | |
| Molar mass | 150.130 g·mol−1 |
| Density | 1.545 g cm−3 |
| Melting point | 108 °C (226 °F; 381 K) |
| Soluble in water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Lyxose is an aldopentose — a monosaccharide containing five carbon atoms, and including an aldehyde functional group. It has chemical formula C5H10O5. It is a C'-2 carbon epimer of the sugar xylose. The name "lyxose" comes from reversing the prefix "xyl" in "xylose".
Lyxose occurs only rarely in nature, for example, as a component of bacterial glycolipids.[1]
References
- ↑ Khoo, K. H.; Dell, Anne; Suzuki, Russell; Morris, Howard R.; McNeil, Michael R.; Brennan, Patrick J.; Besra, Gurdyal S. (10 September 1996). "Chemistry of the Lyxose-Containing Mycobacteriophage Receptors of Mycobacterium phlei/Mycobacterium smegmatis". Biochemistry. American Chemical Society. 35 (36): 11812–11819. doi:10.1021/bi961055+.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.