 | |
| Names | |
|---|---|
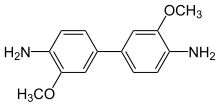
| Preferred IUPAC name
3,3′-Dimethoxy[1,1′-biphenyl]-4,4′-diamine | |
| Other names
3,3'-dimethoxy-4,4’-benzidine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.960 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2811, 2431, 3077 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H16N2O2 | |
| Molar mass | 244.294 g·mol−1 |
| Appearance | White solid |
| Density | 1.178 g/cm3 |
| Melting point | 113 °C (235 °F; 386 K) |
| Boiling point | 356 °C (673 °F; 629 K) |
| 60 mg/L | |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H302, H350 | |
| P201, P202, P264, P270, P281, P301+P312, P308+P313, P330, P405, P501 | |
| Flash point | 206°C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
o-Dianisidine is an organic compound with the formula [(CH3O)(H2N)C6H3]2. A colorless or white solid, it is a bifunctional compound derived via the benzidine rearrangement from o-anisidine.
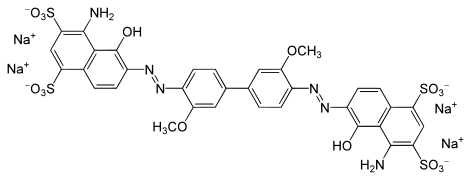
o-Dianisidine is a precursor to some azo dyes by formation of the bis(diazonium) derivative, which is coupled to diverse aromatic compounds. Some commercial dyes derived from o-dianisidine include C. I. Direct Blue 1, 15, 22, 84, and 98.[1]
o-Dianisidine is also used in assaying activity of peroxidase in lab. The general reaction of a peroxidase is as follows.
Where the ROOR' can be hydrogen peroxide, and the electron donor be o-dianisidine.
Safety
The manufacture and degradation of o-dianisidine, like other benzidene derivatives, has attracted regulatory attention.[2] It is also used as a reagent in biochemistry in testing for peroxides.
References
- ↑ Klaus Hunger; Peter Mischke; Wolfgang Rieper; Roderich Raue; Klaus Kunde; Aloys Engel (2005). "Azo Dyes". Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_245. ISBN 3527306730..
- ↑ Golka, Klaus; Kopps, Silke; Myslak, Zdislaw W. (2004). "Carcinogenicity of Azo Colorants: Influence of Solubility and Bioavailability". Toxicology Letters. 151 (1): 203–210. doi:10.1016/j.toxlet.2003.11.016. PMID 15177655.