 | |
| Names | |
|---|---|
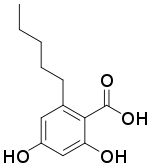
| Preferred IUPAC name
2,4-Dihydroxy-6-pentylbenzoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H16O4 | |
| Molar mass | 224.256 g·mol−1 |
| Related compounds | |
Related compounds |
Cannabidiolic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Olivetolic acid is an organic compound that is an intermediate in the biosynthetic pathway of the cannabinoids in Cannabis sativa.[1]
The ester dimer of olivetolic acid, anziaic acid, is found in lichen.[2]
References
- ↑ Fellermeier, Monika; Zenk, Meinhart H (May 1998). "Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol". FEBS Letters. 427 (2): 283–285. doi:10.1016/S0014-5793(98)00450-5. PMID 9607329.
- ↑ Cheng, Bokun; Cao, Shugeng; Vasquez, Victor; Annamalai, Thirunavukkarasu; Tamayo-Castillo, Giselle; Clardy, Jon; Tse-Dinh, Yuk-Ching (8 April 2013). "Identification of Anziaic Acid, a Lichen Depside from Hypotrachyna sp., as a New Topoisomerase Poison Inhibitor". PLOS ONE. 8 (4): e60770. Bibcode:2013PLoSO...860770C. doi:10.1371/journal.pone.0060770. PMC 3620467. PMID 23593306.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.