 | |
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.854 |
| EC Number |
|
PubChem CID |
|
| |
| |
| Properties | |
| KO4Re | |
| Molar mass | 289.301 g·mol−1 |
| Appearance | white solid[1] |
| Density | 5.39 g·cm−3[1] |
| Melting point | 555[2] °C (1,031 °F; 828 K) |
| Boiling point | 1,370[2] °C (2,500 °F; 1,640 K) |
| 11.9 g·l−1[3] | |
Refractive index (nD) |
1.643 (20 °C)[4] |
| Hazards | |
| GHS labelling:[5] | |
  | |
| Warning | |
| H272, H315, H319, H335 | |
| P210, P220, P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P370+P378, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Potassium perrhenate is an inorganic compound with the chemical formula KReO4.
Preparation
Potassium perrhenate can be produced by the neutralization of potassium hydroxide and perrhenic acid.[2]
Properties
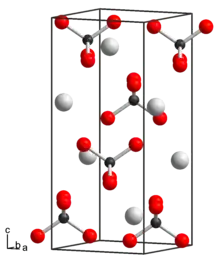
Potassium perrhenate is a white solid that is sparingly soluble in water and ethanol. It have a tetragonal crystal system with the space group I41/a (No. 88), and lattice constants a = 567.4 pm and c = 1266.8 pm.[2] It is a strong oxidizer.[4]
References
- 1 2 Sigma-Aldrich Co., product no. 229822.
- 1 2 3 4 hrsg. von Georg Brauer. Unter Mitarb. von M. Baudler (1981). Handbuch der präparativen anorganischen Chemie / 3 (in German). Stuttgart: Enke. p. 1633. ISBN 3-432-87823-0. OCLC 310719495.
- ↑ Hölemann, Hans; Klesse, Walter (1938-04-22). "Beiträge zur Chemie und Elektrochemie des Rheniums VI. Die Löslichkeit des Kaliumperrhenats in Wasser zwischen 10 und 518°". Zeitschrift für anorganische und allgemeine Chemie. Wiley. 237 (2): 172–176. doi:10.1002/zaac.19382370207. ISSN 0863-1786.
- 1 2 Potassium perrhenate, 99% (metals basis), Re 64% at AlfaAesar, accessed on 2013-08-05 (PDF) (JavaScript required).
- ↑ "Potassium perrhenate". pubchem.ncbi.nlm.nih.gov.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.