 | |
| Clinical data | |
|---|---|
| Other names | Proxibarbital, Centralgol, Ipronal, 5-Allyl-5-(β-hydroxypropyl)barbituric acid |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.018.004 |
| Chemical and physical data | |
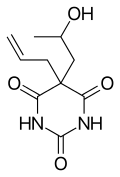
| Formula | C10H14N2O4 |
| Molar mass | 226.232 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Proxibarbital (Ipronal) is a barbiturate derivative synthesized in 1956. It has anti-anxiety properties and is, in contrast to most barbiturates, almost without hypnotic action.[1]
It was also used in the treatment of migraine headaches in a similar manner to butalbital.[2]
Valofane isomerises to Proxibarbal in vivo.
References
- ↑ Zajdel P, Kulig K, Zejc A (2008). Zejc A, Gorczyca M (eds.). Chemia leków, podręcznik dla studentów farmacji i farmaceutów [Drug chemistry, textbook for pharmacy students and pharmacists] (in Polish). Warszawa, Poland. ISBN 978-83-200-3652-7.
{{cite book}}: CS1 maint: location missing publisher (link) - ↑ Sulman FG, Pfeifer Y, Tal E (December 1976). "[Migraine therapy by enzyme induction with proxibarbital]". Therapie der Gegenwart (in German). 115 (12): 2088–103. PMID 14412.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.