 | |
 | |
| Clinical data | |
|---|---|
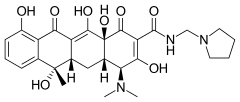
| Other names | (2Z,4S,4aS,5aS,6S,12aS)-4-Dimethylamino-6,10,11,12a-tetrahydroxy-2-[hydroxy-(pyrrolidin-1-ylmethylamino)methylidene]-6-methyl-4,4a,5,5a-tetrahydrotetracene-1,3,12-trione |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.010.938 |
| Chemical and physical data | |
| Formula | C27H33N3O8 |
| Molar mass | 527.574 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Rolitetracycline is a tetracycline antibiotic.[1] Tetracycline is N-Mannich base prodrug that is prepared from tetracycline by condensation with pyrrolidine and formaldehyde to produce rolitetracycline. Rolitetracycline is used as an antibacterial drug, a protein synthesis inhibitor, an antiprotozoal drug and a prodrug.[2][3]
References
- ↑ "Rolitetracycline". PubChem. National Library of Medicine. Retrieved 8 June 2020.
- ↑ PubChem. "Rolitetracycline". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-07-29.
- ↑ Smith L (January 1964). "Rolitetracycline, an antibiotic for parenteral use. (syntetrin, velacycline)". JAMA. 187 (2): 141. doi:10.1001/jama.1964.03060150065017. PMID 14066734.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.