 | |
| Names | |
|---|---|
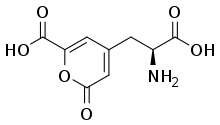
| IUPAC name
4-[(2S)-2-amino-3-hydroxy-3-oxo-propyl]-6-oxo-pyran-2-carboxylic acid | |
| Systematic IUPAC name
4-[(2S)-2-amino-3-hydroxy-3-oxo-propyl]-6-oxo-pyran-2-carboxylic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H9NO6 | |
| Molar mass | 227.172 g·mol−1 |
| Density | 1.604 g/cm3 |
| Melting point | 304.65 °C (580.37 °F; 577.80 K) |
| Boiling point | 528.25 °C (982.85 °F; 801.40 K) at 760 mmHg |
| 2.634e+005 mg/L | |
| Vapor pressure | 1.44E-12 mmHg |
| Hazards | |
| Flash point | 273.2 °C (523.8 °F; 546.3 K) |
| Related compounds | |
Other anions |
stizolobinic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Stizolobic acid is an amino acid found in the sap epicotyl tips of etiolated seedlings of Stizolobium hassjoo.[1]
Biosynthesis
Stizolobium hassjoo catalyzes the conversion of L-dihydroxyphenylalanine into stizolobinic acid, alpha-amino-6-carboxy-2-oxo-2H-pyran-3-propionic acid, and stizolobic acid, alpha-amino-6-carboxy-2-oxo-2H-pyran-4-propionic acid, in the presence of NADP+ or NAD+ under aerobic conditions.
References
- ↑ Hattori, S.; Komamine, A. (1959). "Stizolobic Acid: a New Amino-Acid in Stizolobium hassjoo". Nature. 183 (4668): 1116. Bibcode:1959Natur.183.1116H. doi:10.1038/1831116a0. S2CID 4219696.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.