 | |
| Names | |
|---|---|
| Preferred IUPAC name
Tetramethylthiourea | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.018.626 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H12N2S | |
| Molar mass | 132.23 g·mol−1 |
| Melting point | 78 °C (172 °F; 351 K) |
| Boiling point | 245 °C (473 °F; 518 K) |
| 5,400 mg/l | |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302 | |
| P264, P270, P301+P312, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
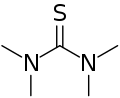
Tetramethylthiourea is a commercially available organic compound used in organic syntheses. The core of the compound is thiourea, with each nitrogen connected to two methyl groups.[1]
References
- ↑ Clovis Peppe; Rafael Pavão das Chagas; Claudio Martins Pereira de Pereira (15 March 2007). "Tetramethylthiourea". Encyclopedia of Reagents for Organic Synthesis Encyclopedia of Reagents for Organic Synthesis, 1. doi:10.1002/9780470842898.rn00711. ISBN 978-0-471-93623-7.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.