 | |
| Names | |
|---|---|
| Preferred IUPAC name
Chlorotri(butyl)stannane | |
| Other names
Tributylchlorotin TBTC | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.014.508 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H27ClSn | |
| Molar mass | 325.51 g·mol−1 |
| Appearance | colorless viscous liquid |
| Density | 1.20 g·cm−3 (20 °C |
| Melting point | −9 °C (16 °F; 264 K) |
| Boiling point | 171 °C (340 °F; 444 K) |
Refractive index (nD) |
1.4903 |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H301, H312, H315, H317, H319, H360FD, H372, H410 | |
| P201, P273, P280, P301+P310+P330, P302+P352+P312, P305+P351+P338 | |
| Flash point | 108 °C (226 °F; 381 K) (closed cup) |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
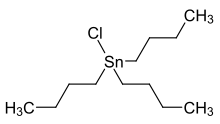
Tributyltin chloride is an organotin compound with the formula (C4H9)3SnCl. It is a colorless liquid that is soluble in organic solvents.
Preparation and reactions
The compound is prepared by a redistribution reaction by combining stannic chloride and tetrabutyltin:
- 3 (C4H9)4Sn + SnCl4 → 4 (C4H9)3SnCl
Tributyltin chloride hydrolyzes to the oxide [(C4H9)3Sn]2O
Tributyltin chloride is used as a precursor to other organotin compounds[1] and reagents, such as tributyltin hydride.
Literature
- ↑ A. F. Renaldo; J. W. Labadie; J. K. Stille (1989). "Palladium-catalyzed Coupling Of Acid Chlorides With Organotin Reagents: Ethyl (E)-4-(4-nitrophenyl)-4-oxo-2-butenoate". Org. Synth. 67: 86. doi:10.15227/orgsyn.067.0086.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.