 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.033.381 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| UI4 | |
| Molar mass | 745.647 g/mol |
| Appearance | black hygroscopic crystals |
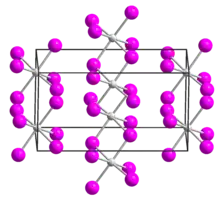
| Structure[1] | |
| monoclinic | |
| C2/c, No. 15 | |
a = 1396.7 pm, b = 847.2 pm, c = 751 pm α = 90°, β = 90.54°, γ = 90° | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Radioactive |
| GHS labelling: | |
   | |
| Danger | |
| H300, H330, H373, H411 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Uranium(IV) iodide, also known as uranium tetraiodide, is an inorganic chemical compound. It is a salt of uranium in oxidation state +4 and iodine.
Preparation
Uranium tetraiodide can be prepared from the reaction between uranium and an excess of iodine.[2]
Properties
Uranium tetraiodide is a black solid and forms needle-like crystals. Upon heating, it dissociates into uranium triiodide and iodine gas.[2] It crystallizes in the monoclinic crystal system, space group C2/c.[1]
References
- 1 2 Levy, J. H.; Taylor, J. C.; Waugh, A. B. (1980). "Crystal structure of uranium(IV) tetraiodide by x-ray and neutron diffraction". Inorganic Chemistry. 19 (3): 672–674. doi:10.1021/ic50205a019. ISSN 0020-1669.
- 1 2 Brauer, Georg (1978). Handbuch der Präparativen Anorganischen Chemie. Vol. II (3rd ed.). Stuttgart: Ferdinand Enke. p. 1218. ISBN 3-432-87813-3.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.