 | |
| Clinical data | |
|---|---|
| Trade names | Intracef, Velocef |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a601206 |
| Routes of administration | Oral, IM, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Well absorbed |
| Protein binding | <10% |
| Metabolism | Nil |
| Elimination half-life | 0.9 hours |
| Excretion | Renal, unchanged |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.049.199 |
| Chemical and physical data | |
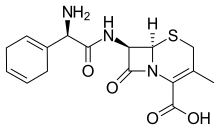
| Formula | C16H19N3O4S |
| Molar mass | 349.41 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 140 to 142 °C (284 to 288 °F) (dec.) |
| |
| |
| (verify) | |
Cefradine (INN) or cephradine (BAN) is a first generation cephalosporin antibiotic.[1]
Indications
- Respiratory tract infections (such as tonsillitis, pharyngitis, and lobar pneumonia) caused by group A beta-hemolytic streptococci and S. pneumoniae (formerly D. pneumonia).[note 1]
- Otitis media caused by group A beta-hemolytic streptococci, S. pneumoniae, H. influenzae, and staphylococci.
- Skin and skin structure infections caused by staphylococci (penicillin-susceptible and penicillin-resistant) and beta-hemolytic streptococci.
- Urinary tract infections, including prostatitis, caused by E. coli, P. mirabilis and Klebsiella species.
Formulations
Cefradine is distributed in the form of capsules containing 250 mg or 500 mg, as a syrup containing 250 mg/5 ml, or in vials for injection containing 500 mg or 1 g.
It is not approved by the FDA for use in the United States.
Synthesis
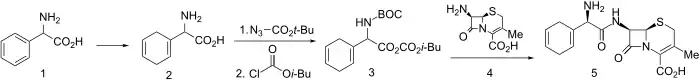
Birch reduction of D-α-phenylglycine led to diene (2). This was N-protected using tert-butoxycarbonylazide and activated for amide formation via the mixed anhydride method using isobutylchloroformate to give 3. Mixed anhydride 3 reacted readily with 7-aminodesacetoxycephalosporanic acid to give, after deblocking, cephradine (5).
Production names
The antibiotic is produced under many brand names across the world.[5]
 Bangladesh: Ancef, Ancef forte, Aphrin, Avlosef, Cefadin, Cephadin, Cephran, Cephran-DS, Cusef, Cusef DS, Dicef , Dicef forte, Dolocef, Efrad, Elocef, Extracef, Extracef-DS, Intracef, Kefdrin, Lebac, Lebac Forte, Medicef, Mega-Cef, Megacin, Polycef, Procef, Procef, Procef forte, Rocef, Rocef Forte DS, Sefin, Sefin DS, Sefnin, Sefrad, Sefrad DS, Sefril, Sefril-DS, Sefro, Sefro-HS, Sephar, Sephar-DS, Septa, Sinaceph, SK-Cef, Sk-Cef DS, Supracef and Supracef-F, Torped, Ultrasef, Vecef, Vecef-DS, Velogen, Sinaceph, Velox
Bangladesh: Ancef, Ancef forte, Aphrin, Avlosef, Cefadin, Cephadin, Cephran, Cephran-DS, Cusef, Cusef DS, Dicef , Dicef forte, Dolocef, Efrad, Elocef, Extracef, Extracef-DS, Intracef, Kefdrin, Lebac, Lebac Forte, Medicef, Mega-Cef, Megacin, Polycef, Procef, Procef, Procef forte, Rocef, Rocef Forte DS, Sefin, Sefin DS, Sefnin, Sefrad, Sefrad DS, Sefril, Sefril-DS, Sefro, Sefro-HS, Sephar, Sephar-DS, Septa, Sinaceph, SK-Cef, Sk-Cef DS, Supracef and Supracef-F, Torped, Ultrasef, Vecef, Vecef-DS, Velogen, Sinaceph, Velox China: Cefradine, Cephradine, Kebili, Saifuding, Shen You, Taididing, Velosef, Xianyi, and Xindadelei
China: Cefradine, Cephradine, Kebili, Saifuding, Shen You, Taididing, Velosef, Xianyi, and Xindadelei Colombia: Cefagram, Cefrakov, Cefranil , Cefrex, and Kliacef
Colombia: Cefagram, Cefrakov, Cefranil , Cefrex, and Kliacef Egypt: Cefadrin, Cefadrine, Cephradine, Cephraforte, Farcosef, Fortecef, Mepadrin, Ultracef, and Velosef
Egypt: Cefadrin, Cefadrine, Cephradine, Cephraforte, Farcosef, Fortecef, Mepadrin, Ultracef, and Velosef France: Dexef
France: Dexef Hong Kong: Cefradine and ChinaQualisef-250
Hong Kong: Cefradine and ChinaQualisef-250 Indonesia: Dynacef, Velodine, and Velodrom
Indonesia: Dynacef, Velodine, and Velodrom Lebanon: Eskacef, Julphacef, and Velosef
Lebanon: Eskacef, Julphacef, and Velosef Lithuania: Tafril
Lithuania: Tafril Myanmar: Sinaceph
Myanmar: Sinaceph Oman: Ceframed, Eskasef, Omadine, and Velocef
Oman: Ceframed, Eskasef, Omadine, and Velocef Pakistan: Abidine, Ada-Cef, Ag-cef, Aksosef, Amspor, Anasef, Antimic, Atcosef, Bactocef, Biocef, Biodine, Velora, Velosef
Pakistan: Abidine, Ada-Cef, Ag-cef, Aksosef, Amspor, Anasef, Antimic, Atcosef, Bactocef, Biocef, Biodine, Velora, Velosef Peru: Abiocef, Cefradinal, Cefradur, Cefrid, Terbodina II, Velocef, Velomicin
Peru: Abiocef, Cefradinal, Cefradur, Cefrid, Terbodina II, Velocef, Velomicin Philippines: Altozef, Racep, Senadex, Solphride, Yudinef, Zefadin, Zefradil, and Zolicef
Philippines: Altozef, Racep, Senadex, Solphride, Yudinef, Zefadin, Zefradil, and Zolicef Poland: Tafril
Poland: Tafril Portugal: Cefalmin, Cefradur
Portugal: Cefalmin, Cefradur South Africa: Cefril A
South Africa: Cefril A South Korea: Cefradine and Tricef
South Korea: Cefradine and Tricef Taiwan: Cefadin, Cefamid, Cefin, Cekodin, Cephradine, Ceponin, Lacef, Licef-A, Lisacef, Lofadine, Recef, S-60, Sefree, Sephros, Topcef, Tydine, Unifradine, and U-Save
Taiwan: Cefadin, Cefamid, Cefin, Cekodin, Cephradine, Ceponin, Lacef, Licef-A, Lisacef, Lofadine, Recef, S-60, Sefree, Sephros, Topcef, Tydine, Unifradine, and U-Save UK: Cefradune (Kent)
UK: Cefradune (Kent) Vietnam: Eurosefro and Incef
Vietnam: Eurosefro and Incef
See also
- Cephapirin
- Cephacetrile
- Cefamandole
- Ampicillin (Has the same chemical formula)
Notes
- ↑ Penicillin is the usual drug of choice in the treatment and prevention of streptococcal infections, including the prophylaxis of rheumatic fever. Cefuroxime is generally effective in the eradication of streptococci from the nasopharynx
References
- ↑ British National Formulary (45 ed.). London: British Medical Association. 2003.
- ↑ Dolfini JE, Applegate HE, Bach G, Basch H, Bernstein J, Schwartz J, Weisenborn FL (February 1971). "A new class of semisynthetic penicillins and cephalosporins derived from D-2-(1,4-cyclohexadienyl)glycine". Journal of Medicinal Chemistry. 14 (2): 117–9. doi:10.1021/jm00284a008. PMID 5544394.
- ↑ U.S. Patent 3,485,819
- ↑ DE 1931722, Weisenborn, Frank L.; Dolfini, Joseph E. & Bach, Georges G. et al., "α-Amino-cyclohexadienyl-alkylen-penicilline und -cephalosporine, ihre Salze, und Verfahren zu ihrer Herstellung [Alpha-amino-cyclohexadienyl-alkylene-penicillins and cephalosporins, their salts, and processes for their preparation]", published 1970-01-08, assigned to E. R. Squibb & Sons Inc.
- ↑ "Cefradine". Retrieved 5 May 2016.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.