 | |
| Clinical data | |
|---|---|
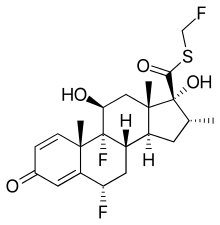
| Other names | 6α,9α-Difluoro-11β,17α-dihydroxy-16α-methyl-21-thia-21-fluoromethylpregna-1,4-dien-3,20-dione; S-(Fluoromethyl)-6α,9α-difluoro-11β,17α-dihydroxy-16α-methyl-3-oxoandrosta-1,4-diene-17β-carbothioate |
| Routes of administration | Intranasal, inhaled, topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 0.51% (Intranasal) |
| Protein binding | 91.0% |
| Metabolism | Intranasal Liver (CYP3A4-mediated) |
| Elimination half-life | 10 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H27F3O4S |
| Molar mass | 444.51 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fluticasone is a manufactured glucocorticoid used to treat nasal symptoms.[1][2][3][4][5] Both the esters, fluticasone propionate (a brand name for which is Flovent) and fluticasone furoate, are also used as topical anti-inflammatories and inhaled corticosteroids, and are used much more commonly in comparison.[3][2][4][6]
It is on the World Health Organization's List of Essential Medicines.[7] In 2021, it was the 23rd most commonly prescribed medication in the United States, with more than 25 million prescriptions,[8][9] although it is also sold over the counter.[10]
See also
References
- ↑ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 574–. ISBN 978-1-4757-2085-3.
- 1 2 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 1337–. ISBN 978-3-88763-075-1.
- 1 2 Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 124–. ISBN 978-94-011-4439-1.
- 1 2 "Fluticasone - FDA prescribing information, side effects and uses".
- ↑ Briggs GG, Freeman RK, Yaffe SJ (2012). Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins. p. 600. ISBN 978-1451153590.
- ↑ Spratto GR, Woods AL (2012). Delmar Nurse's Drug Handbook 2012. Cengage Learning. p. 748. ISBN 978-1111310653.
- ↑ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ↑ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ↑ "Fluticasone - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.
- ↑ "Fluticasone Nasal Spray". Retrieved 21 October 2022.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.